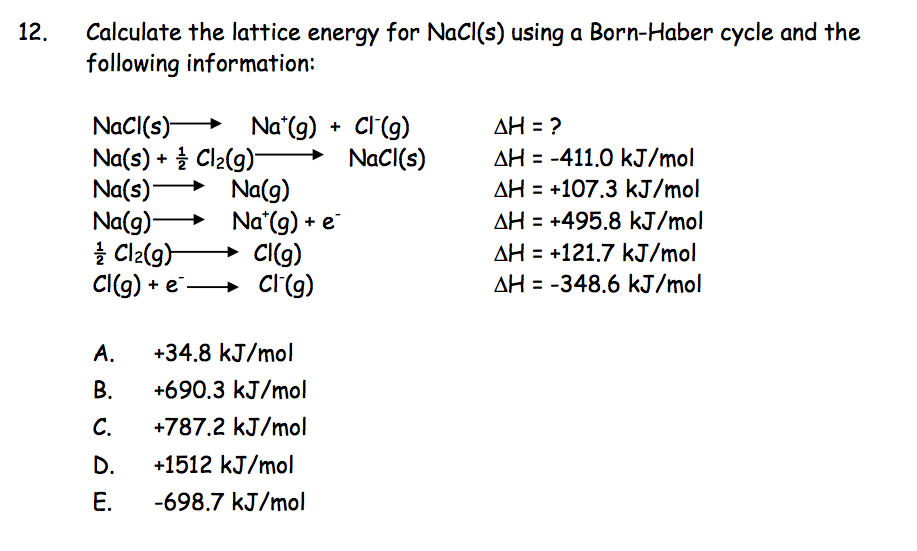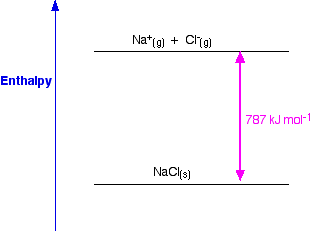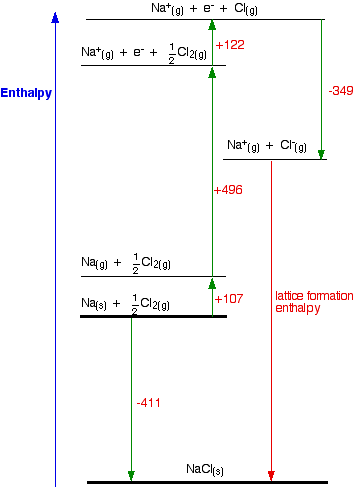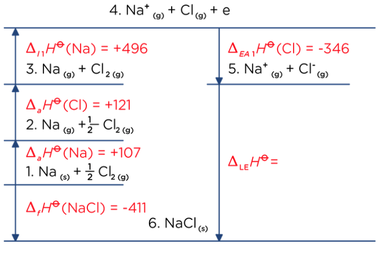
Born Haber Cycle, Basic Introduction, Lattice Energy, Hess Law & Enthalpy of Formation - Chemistry - YouTube

Calculate the lattice enthalpy of KCl from the following data by Born- Haber's Cycle. Enthalpy of sublimation of K = 89 kJ mol^(–1) Enthalpy of dissociation of Cl = 244 kJ mol^(–1)

SOLVED: Use the data provided below to calculate the lattice energy of RbCL Is this value greater or less than the lattice energy of NaCl? Explain Electron affinity of Cl = -349

Calculations using Born-Haber Cycles (5.1.4) | CIE A Level Chemistry Revision Notes 2022 | Save My Exams

SOLVED:Calculate the lattice energy of sodium oxide (Na2 O) from the following data: Ionization energy of Na(g)=495 kJ / mol Electron affinity of O(g) for 2 electrons =603 kJ / mol Energy

How do you calculate the lattice energy of NaF using the Born-Haber cycle? (Delta Hsub for sodium is 107.5 kJ/mol) | Homework.Study.com